Tämä on Wikipediasta. Siitä ei ole suomalaista versiota:
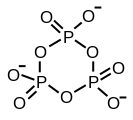
Triphosphates are salts or esters of polymeric oxyanions formed from tetrahedral PO4 (phosphate) structural units linked together by sharing oxygen atoms. When two corners are shared the polyphosphate may have a linear chain structure or a cyclic ring structure. In biology the polyphosphate esters AMP, ADP and ATP are involved in energy transfer. A variety of polyphosphates find application in mineral sequestration in municipal waters, generally being present at 1 to 5 pm.[1] GTP, CTP, and UTP are also nucleotides important in the protein synthesis, lipid synthesis and carbohydrate metabolism, respectively.
Contents
Structure & Formation
-
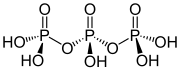
Structure of triphosphoric acid
-
Adenosine diphosphate (ADP)
Sharing of three corners is possible as in the sheet-structure Phyllosilicates, but such structures occur only under extreme conditions. Three-corner sharing also occurs in phosphorus pentoxide, P4O10, which has a 3-dimensional structure.
Chemically, the polymerization reaction can be seen as a condensation reaction. The process begins with two phosphate units coming together.
- 2 PO43− + 2 H+
 P2O74− + H2O
P2O74− + H2O
Acid-base and complexation properties
Polyphosphates are weak bases. A lone pair of electrons on an oxygen atom can be donated to a hydrogen ion (proton) or a metal ion in a typical Lewis acid-Lewis base interaction. This has profound significance in biology. For instance, adenosine triphosphate (ATP) is about 25% protonated in aqueous solution at pH 7.[2]- ATP4- + H+
 ATPH3-, pKa
ATPH3-, pKa  6.6
6.6
ATP forms chelate complexes with metal ions. The stability constant for the equilibrium
- ATP4- + Mg2+
 MgATP2-, log β
MgATP2-, log β  4
4
The "high energy" phosphate bond
The energy released in ATP hydrolysis,- ATP4- + H2O → ADP3- + Pi-
 -36.8 kJ mol−1 is large by biological standards. Pi
stands for inorganic phosphate, which is protonated at biological pH.
However, it is not large by inorganic standards. The term "high energy"
refers to the fact that it is high relative to the amount of energy
released in the organic chemical reactions that can occur in living systems.
-36.8 kJ mol−1 is large by biological standards. Pi
stands for inorganic phosphate, which is protonated at biological pH.
However, it is not large by inorganic standards. The term "high energy"
refers to the fact that it is high relative to the amount of energy
released in the organic chemical reactions that can occur in living systems.High-polymeric inorganic polyphosphates
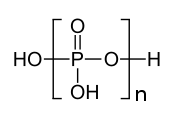
High-polymeric inorganic polyphosphates were found in living organisms by L. Liberman in 1890. These compounds are linear polymers containing a few to several hundred residues of orthophosphate linked by energy-rich phosphoanhydride bonds.Previously, it was considered either as “molecular fossil” or as only a phosphorus and energy source providing the survival of microorganisms under extreme conditions. These compounds are now known to also have regulatory roles, and to occur in representatives of all kingdoms of living organisms, participating in metabolic correction and control on both genetic and enzymatic levels. Polyphosphate is directly involved in the switching-over of the genetic program characteristic of the exponential growth stage of bacteria to the program of cell survival under stationary conditions, “a life in the slow line”. They participate in many regulatory mechanisms occurring in bacteria:
- They participate in the induction of rpoS, an RNA-polymerase subunit which is responsible for the expression of a large group of genes involved in adjustments to the stationary growth phase and many stressful agents.
- They are important for cell motility, biofilms formation and virulence.
- Polyphosphates and exopolyphosphatases participate in the regulation of the levels of the stringent response factor, guanosine 5'-diphosphate 3'-diphosphate (ppGpp), a second messenger in bacterial cells.
- Polyphosphates participate in the formation of channels across the living cell membranes. The above channels formed by polyphosphate and poly-b-hydroxybutyrate with Ca2+ are involved in the transport processes in a variety of organisms.
- An important function of polyphosphate in microorganisms—prokaryotes and the lower eukaryotes—is to handle changing environmental conditions by providing phosphate and energy reserves. Polyphosphates are present in animal cells, and there are many data on its participation in the regulatory processes during development and cellular proliferation and differentiation—especially in bone tissues and brain.


Inga kommentarer:
Skicka en kommentar