LOCUS NP_000361 278 aa linear PRI 15-MAR-2015
DEFINITION alpha-tocopherol transfer protein [Homo sapiens].
ACCESSION NP_000361 XP_001128514
VERSION NP_000361.1 GI:4507723
DBSOURCE REFSEQ: accession NM_000370.3
KEYWORDS RefSeq.
SOURCE Homo sapiens (human)
ORGANISM Homo sapiens
Eukaryota; Metazoa; Chordata; Craniata; Vertebrata; Euteleostomi;
Mammalia; Eutheria; Euarchontoglires; Primates; Haplorrhini;
Catarrhini; Hominidae; Homo.
REFERENCE 1 (residues 1 to 278)
AUTHORS Kono N, Ohto U, Hiramatsu T, Urabe M, Uchida Y, Satow Y and Arai H.
TITLE Impaired alpha-TTP-PIPs interaction underlies familial vitamin E
deficiency
JOURNAL Science 340 (6136), 1106-1110 (2013)
PUBMED 23599266
REMARK GeneRIF: The crystal structure of the
alpha-TTP-phosphatidylinositol phosphates (PIPs) complex revealed
that the familial vitamin E deficiency-related arginine residues
interacted with phosphate groups of the PIPs and that the PIPs
binding caused the lid of the alpha-tocopherol-binding pocket to
open.
REFERENCE 2 (residues 1 to 278)
AUTHORS Etzl RP, Vrekoussis T, Kuhn C, Schulze S, Poschl JM, Makrigiannakis
A, Jeschke U and Rotzoll DE.
TITLE Oxidative stress stimulates alpha-tocopherol transfer protein in
human trophoblast tumor cells BeWo
JOURNAL J Perinat Med 40 (4), 373-378 (2012)
PUBMED 22752767
REMARK GeneRIF: study demonstrated that alpha -TTP can be upregulated in
case of oxidative stress in BeWo trophoblast cells; speculate that
possibly, alpha -TTP is notonly involved in normal pregnancy, but
also in cases of pregnancy disorders with intense oxidative stress
Publication Status: Online-Only
REFERENCE 3 (residues 1 to 278)
AUTHORS Zhang WX, Thakur V, Lomize A, Pogozheva I, Panagabko C, Cecchini M,
Baptist M, Morley S, Manor D and Atkinson J.
TITLE The contribution of surface residues to membrane binding and ligand
transfer by the alpha-tocopherol transfer protein (alpha-TTP)
JOURNAL J. Mol. Biol. 405 (4), 972-988 (2011)
PUBMED 21110980
REMARK GeneRIF: Substitution of residues in helices A8 (F165A and F169A)
and A10 (I202A, V206A and M209A) decreased the rate of
intermembrane ligand transfer as well as protein adsorption to
phospholipid bilayers.
REFERENCE 4 (residues 1 to 278)
AUTHORS Booij JC, Bakker A, Kulumbetova J, Moutaoukil Y, Smeets B, Verheij
J, Kroes HY, Klaver CC, van Schooneveld M, Bergen AA and Florijn
RJ.
TITLE Simultaneous mutation detection in 90 retinal disease genes in
multiple patients using a custom-designed 300-kb retinal
resequencing chip
JOURNAL Ophthalmology 118 (1), 160-167 (2011)
PUBMED 20801516
REMARK GeneRIF: Observational study of genetic testing. (HuGE Navigator)
REFERENCE 5 (residues 1 to 278)
AUTHORS Morley S, Thakur V, Danielpour D, Parker R, Arai H, Atkinson J,
Barnholtz-Sloan J, Klein E and Manor D.
TITLE Tocopherol transfer protein sensitizes prostate cancer cells to
vitamin E
JOURNAL J. Biol. Chem. 285 (46), 35578-35589 (2010)
PUBMED 20826775
REMARK GeneRIF: Data show that reduction ('knockdown') of tocopherol
transfer protein (TTP) expression resulted in resistance to the
vitamin E.
REFERENCE 6 (residues 1 to 278)
AUTHORS Gotoda T, Arita M, Arai H, Inoue K, Yokota T, Fukuo Y, Yazaki Y and
Yamada N.
TITLE Adult-onset spinocerebellar dysfunction caused by a mutation in the
gene for the alpha-tocopherol-transfer protein
JOURNAL N. Engl. J. Med. 333 (20), 1313-1318 (1995)
PUBMED 7566022
REFERENCE 7 (residues 1 to 278)
AUTHORS Arita M, Sato Y, Miyata A, Tanabe T, Takahashi E, Kayden HJ, Arai H
and Inoue K.
TITLE Human alpha-tocopherol transfer protein: cDNA cloning, expression
and chromosomal localization
JOURNAL Biochem. J. 306 (PT 2), 437-443 (1995)
PUBMED 7887897
REFERENCE 8 (residues 1 to 278)
AUTHORS Ouahchi K, Arita M, Kayden H, Hentati F, Ben Hamida M, Sokol R,
Arai H, Inoue K, Mandel JL and Koenig M.
TITLE Ataxia with isolated vitamin E deficiency is caused by mutations in
the alpha-tocopherol transfer protein
JOURNAL Nat. Genet. 9 (2), 141-145 (1995)
PUBMED 7719340
REFERENCE 9 (residues 1 to 278)
AUTHORS Ben Hamida C, Doerflinger N, Belal S, Linder C, Reutenauer L, Dib
C, Gyapay G, Vignal A, Le Paslier D and Cohen D.
CONSRTM et al
TITLE Localization of Friedreich ataxia phenotype with selective vitamin
E deficiency to chromosome 8q by homozygosity mapping
JOURNAL Nat. Genet. 5 (2), 195-200 (1993)
PUBMED 8252047
REFERENCE 10 (residues 1 to 278)
AUTHORS Schuelke,M.
TITLE Ataxia with Vitamin E Deficiency
JOURNAL (in) Pagon RA, Adam MP, Ardinger HH, Bird TD, Dolan CR, Fong CT,
Smith RJH and Stephens K (Eds.);
GENEREVIEWS(R);
(1993)
PUBMED 20301419
COMMENT REVIEWED REFSEQ: This record has been curated by NCBI staff. The
reference sequence was derived from BC058000.1, BC041784.1 and
AC120042.3.
This sequence is a reference standard in the RefSeqGene project.
On Sep 20, 2006 this sequence version replaced gi:113420428.
Summary: This gene encodes a soluble protein that binds
alpha-tocopherol, a form of vitamin E, with high selectivity and
affinity. This protein plays an important role in regulating
vitamin E levels in the body by transporting vitamin E between
membrane vesicles and facilitating the secretion of vitamin E from
hepatocytes to circulating lipoproteins. Mutations in this gene
cause hereditary vitamin E deficiency (ataxia with vitamin E
deficiency, AVED) and retinitis pigmentosa. [provided by RefSeq,
Nov 2009].
Sequence Note: This RefSeq record was created from transcript and
genomic sequence data to make the sequence consistent with the
reference genome assembly. The genomic coordinates used for the
transcript record were based on transcript alignments.
Publication Note: This RefSeq record includes a subset of the
publications that are available for this gene. Please see the Gene
record to access additional publications.
##Evidence-Data-START##
Transcript exon combination :: BC058000.1, BC041784.1 [ECO:0000332]
RNAseq introns :: single sample supports all introns
SAMEA1968540, SAMEA1970526
[ECO:0000348]
##Evidence-Data-END##
FEATURES Location/Qualifiers
source 1..278
/organism="Homo sapiens"
/db_xref="taxon:9606"
/chromosome="8"
/map="8q12.3"
Protein 1..278
/product="alpha-tocopherol transfer protein"
/note="tocopherol (alpha) transfer protein (ataxia
(Friedreich-like) with vitamin E deficiency); alpha-TTP"
/calculated_mol_wt=31619
Region <40 ..73="" cdd="" cddsrv.cgi="" db_xref="CDD:<a href=" http:="" note="CRAL/TRIO, N-terminal domain; smart01100" region_name="CRAL_TRIO_N" tructure="" uid="215024" www.ncbi.nlm.nih.gov="">215024
"
Region 95..248
/region_name="SEC14"
/note="Sec14p-like lipid-binding domain. Found in
secretory proteins, such as S. cerevisiae
phosphatidylinositol transfer protein (Sec14p), and in
lipid regulated proteins such as RhoGAPs, RhoGEFs and
neurofibromin (NF1). SEC14 domain of Dbl is known to...;
cd00170"
/db_xref="CDD:238099"
Site order(113,115,117,143,158,160,176,180,184,188,191,194,196,
203,210,222)
/site_type="other"
/note="phospholipid binding pocket [chemical binding]"
/db_xref="CDD:238099"
Site order(189,221)
/site_type="other"
/note="salt bridge"
/db_xref="CDD:238099"
CDS 1..278
/gene="TTPA"
/gene_synonym="alphaTTP; ATTP; AVED; TTP1"
/coded_by="NM_000370.3:33..869"
/db_xref="CCDS:CCDS6178.1"
/db_xref="GeneID:7274"
/db_xref="HGNC:HGNC:12404"
/db_xref="HPRD:02685"
/db_xref="MIM:600415"
ORIGIN
1
maearsqpsa gpqlnalpdh spllqpglaa lrrrareagv plaplpltds fllrflrard
61
fdldlawrll knyykwraec peisadlhpr siigllkagy hgvlrsrdpt gskvliyria
121
hwdpkvftay dvfrvslits elivqevetq rngikaifdl egwqfshafq itpsvakkia
181
avltdsfplk vrgihlinep vifhavfsmi kpfltekike rihmhgnnyk qsllqhfpdi
241
lpleyggeef smedicqewt nfimksedyl ssisesiq
//

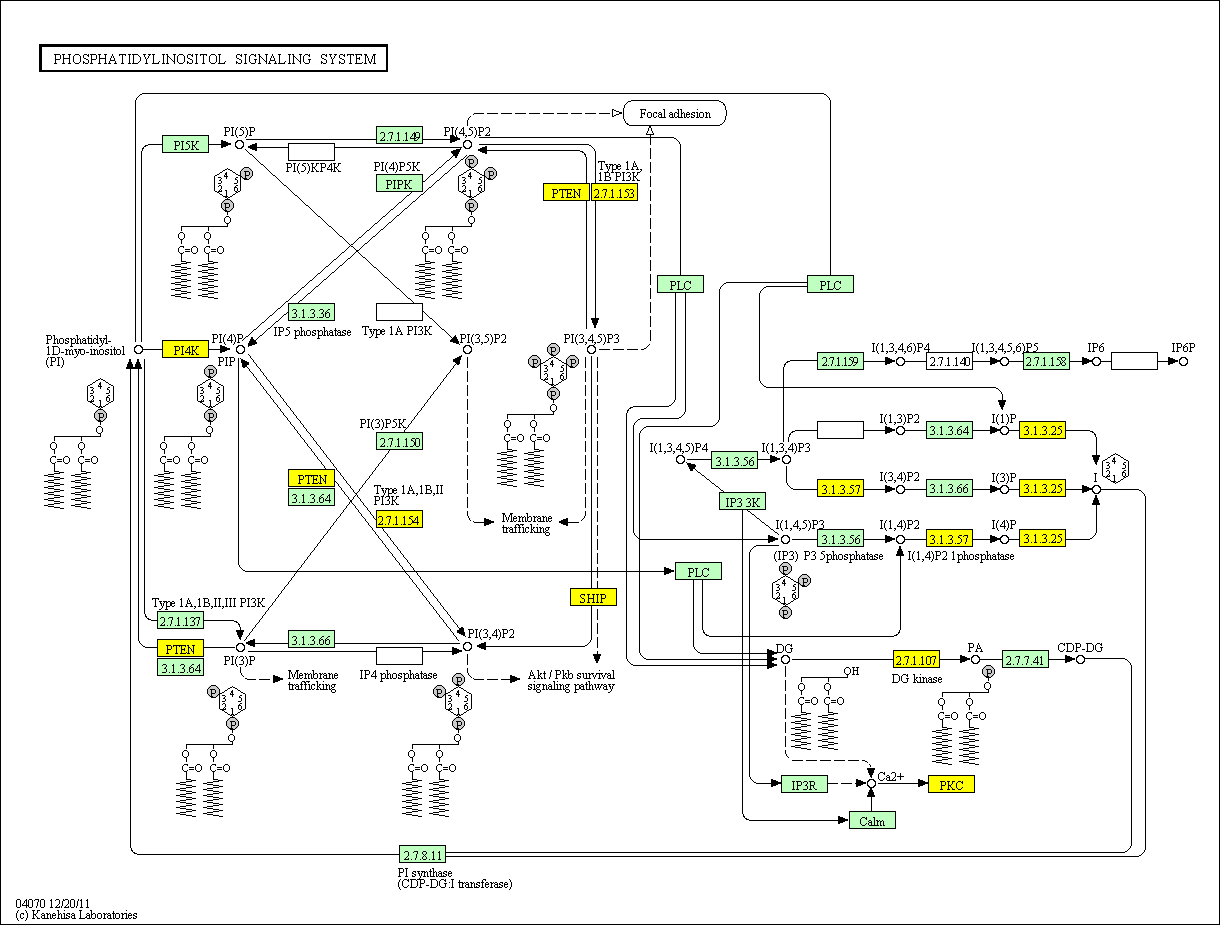
 In a recent paper in Cell
143, 897-910 2010, Chakraborty
et. al. report a new player in the insulin story. This is reviewed in the
same issue of Cell by Brendan D. Manning Cell 143 861-862 2010 (DOI 10.1016/j.cell.2010.11.040).
They have discovered an insulin receptor product which has not been reported
earlier. Activation of the insulin receptor stimulates both
phosphorylation of IRS1 and activation of inositol hexakisphosphate (IP6) kinase
(IP6K1). The latter yields 5-diphospho-inositolpentakisphosphate (5-PP-IP5
or IP7). IP7 binds to Akt, inactivating it and preventing its function as a substrate for mTORC2* and PDK1
phosphorylation. That is, insulin produces signal substances that both
activate and deactivate Akt. The "on" signal is the PIP2-PIP3 sequence.
The "off" signal is the IP6-IP7 sequence. The balance between these may dominate
insulin regulation of metabolism. Perhaps even more important is the
possibility that the key to understanding "insulin resistance" may lie in the
balance between these.
In a recent paper in Cell
143, 897-910 2010, Chakraborty
et. al. report a new player in the insulin story. This is reviewed in the
same issue of Cell by Brendan D. Manning Cell 143 861-862 2010 (DOI 10.1016/j.cell.2010.11.040).
They have discovered an insulin receptor product which has not been reported
earlier. Activation of the insulin receptor stimulates both
phosphorylation of IRS1 and activation of inositol hexakisphosphate (IP6) kinase
(IP6K1). The latter yields 5-diphospho-inositolpentakisphosphate (5-PP-IP5
or IP7). IP7 binds to Akt, inactivating it and preventing its function as a substrate for mTORC2* and PDK1
phosphorylation. That is, insulin produces signal substances that both
activate and deactivate Akt. The "on" signal is the PIP2-PIP3 sequence.
The "off" signal is the IP6-IP7 sequence. The balance between these may dominate
insulin regulation of metabolism. Perhaps even more important is the
possibility that the key to understanding "insulin resistance" may lie in the
balance between these.